Multi-omics analysis of innate and adaptive responses to BCG vaccination reveals epigenetic cell states that predict trained immunity
Supplemental Website
Study overview
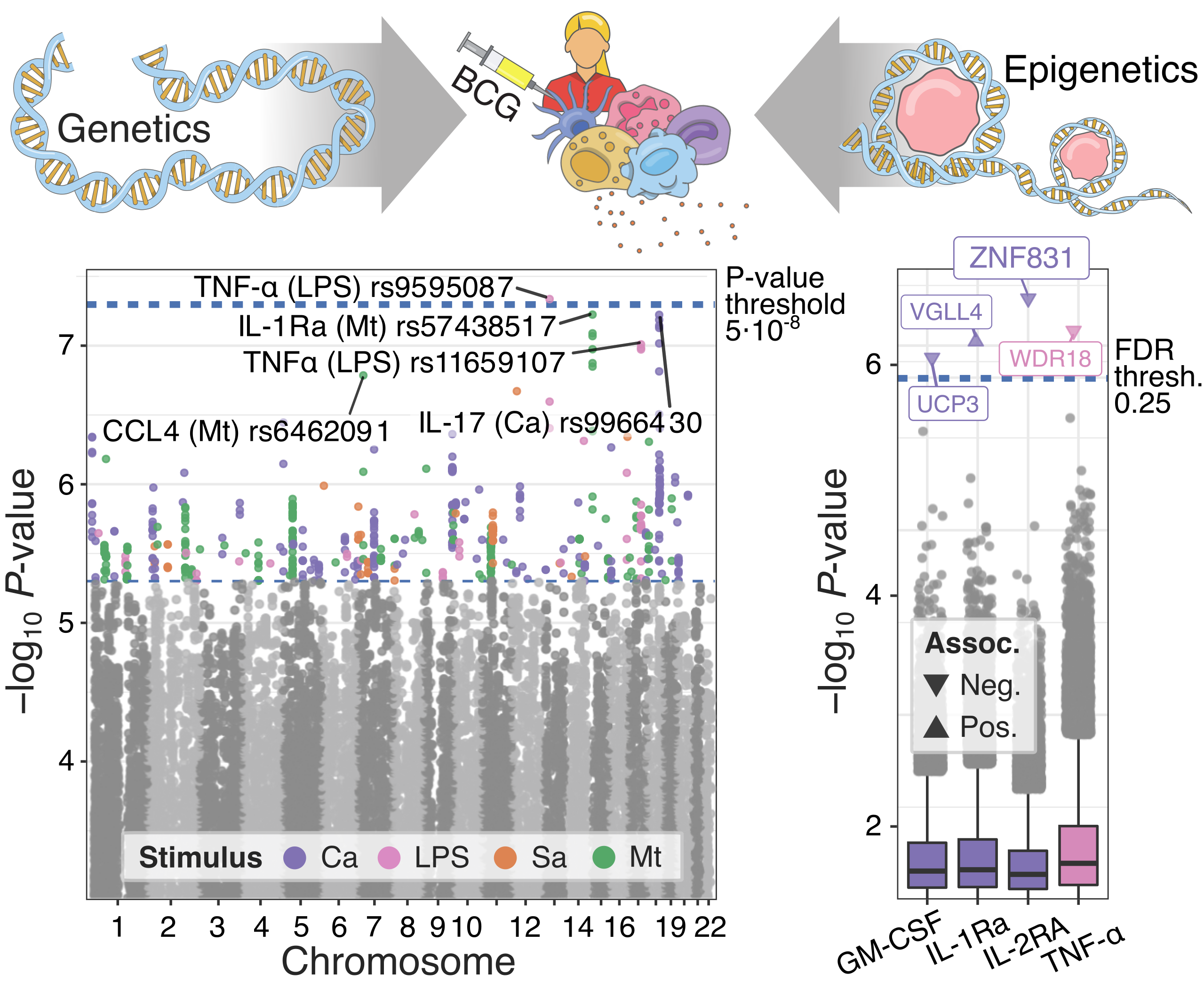
Genome-wide association studies chart genetic and epigenetic regulation of immune function and BCG responses.
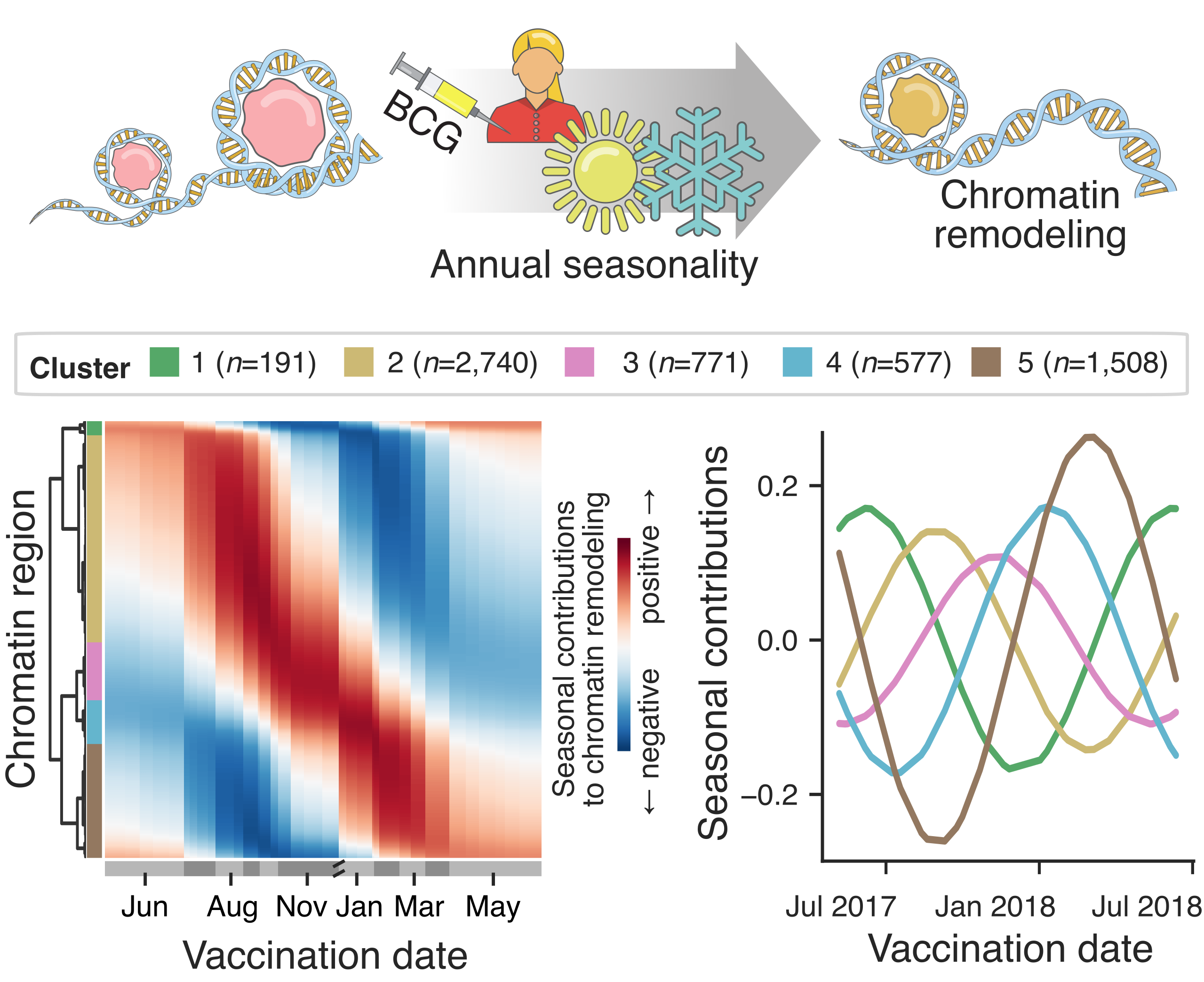
Magnitude of BCG-induced changes in chromatin accessibility is subject to annual seasonal variability.
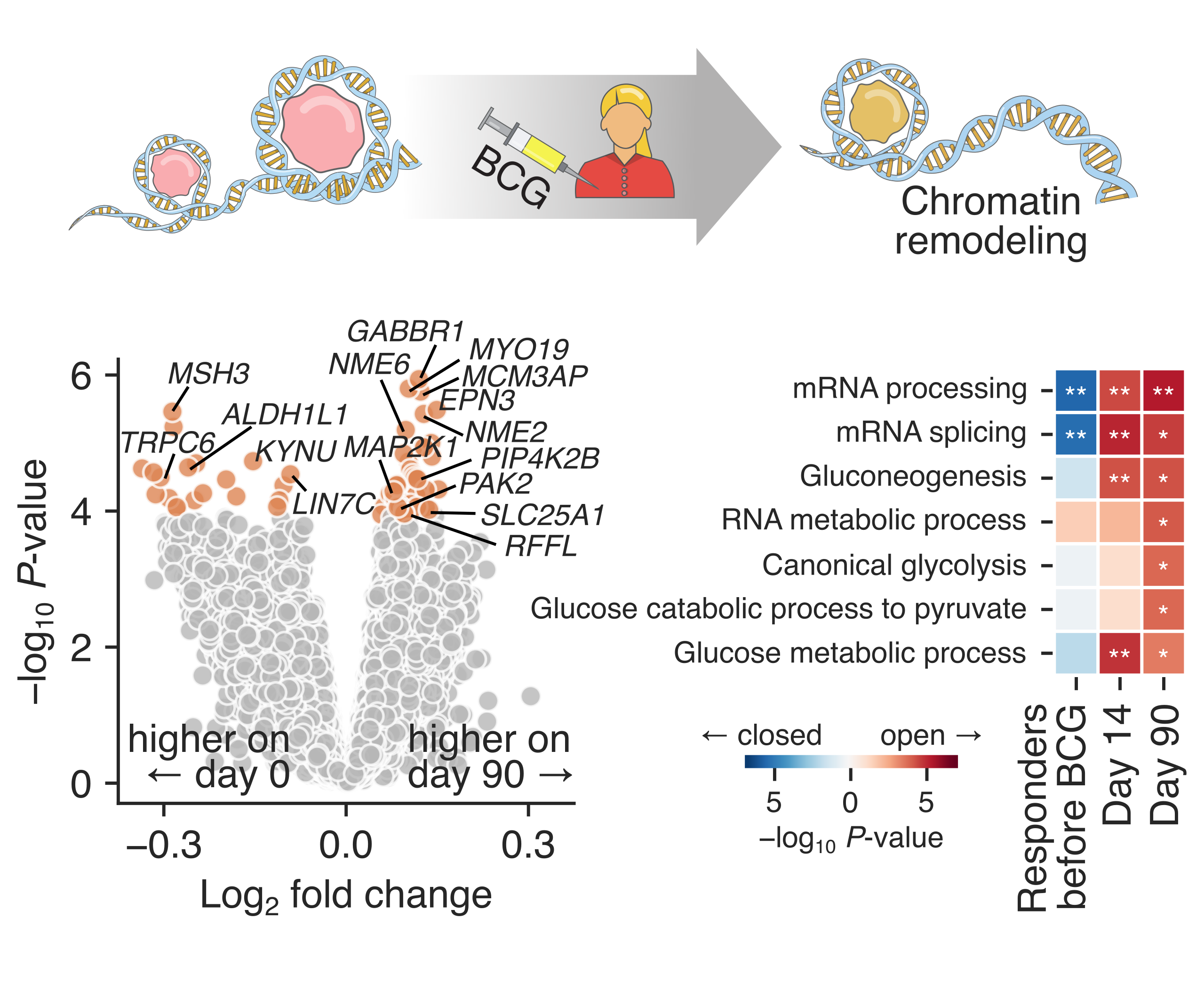
BCG vaccination induces chromatin remodeling associated with innate immune memory.

Baseline epigenetic cell state predicts trained immunity responders.
Data
| Description | Links |
|---|---|
|
Genome Browser Tracks |
GRCh38, all samples, fully customizable: UCSC Track Hub |
| Processed Datasets |
ATAC-seq count data: GEO Series GSE241092 Raw ATAC-seq data: EGA EGAS50000000090 Immunological phenotyping data (immune cell frequency, circulating inflammatory markers, cytokine production capacity): Zenodo record 10.5281/zenodo.10288920 |
| Supplemental Tables |
Table S1: Annotation of individuals and samples in the 300BCG cohort; ATAC-seq quality statistics; annotation of the filtered consensus set of genomic regions; scRNA-seq quality statistics
(XLSX)
Table S2: Immune parameters (immune cell frequency, circulating inflammatory markers, cytokine production capacity) associated with host factors and BCG vaccination; BCG-induced changes in cytokine production capacity associated with host factors, annual seasonality, and immune parameters at baseline (XLSX) Table S3: Genomic regions associated with host factors, with circulating inflammatory markers and cytokine production capacity at baseline, and with BCG-induced changes in cytokine production capacity; gene set enrichments of genomic regions associated with host factors, with circulating inflammatory markers and cytokine production capacity at baseline, and with BCG-induced changes in cytokine production capacity (XLSX) Table S4: Genetic variants (SNPs) associated with circulating inflammatory markers and cytokine production capacity at baseline, and with BCG-induced changes in cytokine production capacity; Gene set enrichments of genes linked to SNPs associated with circulating inflammatory markers and cytokine production capacity at baseline, and with BCG-induced changes in cytokine production capacity (XLSX) Table S5: Variance partitioning for immune parameters (circulating inflammatory markers and cytokine produc-tion capacity at baseline, BCG-induced changes in cytokine production capacity), and; overlap between the genetic associations with the immune parameters and the epigenetic associations with the immune parameters (XLSX) Table S6: Genomic regions associated with BCG-induced chromatin remodeling and its seasonal variability, and with BCG responder status at baseline; genes associated with BCG-induced changes in gene expression (with and without LPS stimulation), and with BCG-induced epigenetic potential; gene set enrichments of genomic regions associated with BCG-induced chromatin remodeling and its seasonal variability, and with BCG responder status at baseline; genes associated with BCG-induced changes in gene expression (with and without LPS stimulation), and with BCG-induced epigenetic potential; region set enrichments of genomic regions associated with BCG-induced chromatin remodeling and its seasonal variability, and with BCG responder status at baseline (XLSX) Table S7: List of public trained immunity datasets that were used to create a reference database of trained immunity genes and genomic regions, and single-cell RNA-seq validation (XLSX) |
| Extended Supplement |
Ext. Table S1.1:
Annotation of all genomic regions (without filtering)
(XLSX)
Ext. Table S3.1: Genomic regions associated with host factors at baseline (complete set of results) (XLSX) Ext. Table S3.2: Genomic regions associated with circulating inflammatory markers at baseline (complete set of results) (view files) Ext. Table S3.3: Genomic regions associated with cytokine production capacity at baseline (complete set of results) (view files) Ext. Table S3.4: Genomic regions whose chromatin accessibility at baseline is associated with BCG-induced changes in cytokine production capacity 14 days after vaccination (complete set of results) (view files) Ext. Table S3.5: Genomic regions whose chromatin accessibility at baseline is associated with BCG-induced changes in cytokine production capacity 90 days after vaccination (complete set of results) (view files) Ext. Table S3.6: Gene set enrichments of putative promoters associated with host factors, circulating inflammatory markers and cytokine production capacity at baseline, and BCG-induced changes in cytokine production capacity (XLSX) Ext. Table S3.7: Region set enrichments of genomic regions associated with host factors, circulating inflammatory markers and cytokine production capacity at baseline, and BCG-induced changes in cytokine production capacity (XLSX) Ext. Table S4.1: SNPs associated with circulating inflammatory markers at baseline (complete set of results) (view files) Ext. Table S4.2: SNPs associated with cytokine production capacity at baseline (complete set of results) (view files) Ext. Table S4.3: SNPs associated with BCG-induced changes in cytokine production capacity 14 days after vaccination (complete set of results) (view files) Ext. Table S4.4: SNPs associated with BCG-induced changes in cytokine production capacity 90 days after vaccination (complete set of results) (view files) Ext. Table S6.1: Genomic regions associated with BCG-induced chromatin remodeling and its seasonal variability, and BCG responders at baseline (complete set of results) (XLSX) Ext. Table S6.2: Gene set enrichments of putative promoters associated with BCG-induced chromatin remodeling and its seasonal variability, and BCG responders at baseline (XLSX) Ext. Table S7.1: Trained immunity associated genes and genomic regions mapped into the genomic region space that we used in this study (complete set and metadata) (view files) |
| Source Code |
ATAC-seq processing pipeline: 300BCG ATACseq pipeline
Data analysis: 300BCG analysis |
Citation
Simone J.C.F.M. Moorlag*, Lukas Folkman*, Rob ter Horst*, Thomas Krausgruber*, Daniele Barreca, Linda C. Schuster, Victoria Fife, Vasiliki Matzaraki, Wenchao Li, Stephan Reichl, Vera P. Mourits, Valerie A.C.M. Koeken, L. Charlotte J. de Bree, Helga Dijkstra, Heidi Lemmers, Bram van Cranenbroek, Esther van Rijssen, Hans J.P.M. Koenen, Irma Joosten, Cheng-Jian Xu, Yang Li, Leo A.B. Joosten, Reinout van Crevel, Mihai G. Netea#, and Christoph Bock#^.
Multi-omics analysis of innate and adaptive responses to BCG vaccination reveals epigenetic cell states that predict trained immunity. Immunity. 2024; 57(1). doi: 10.1016/j.immuni.2023.12.005
* These authors contributed equally
# Senior author
^ Lead contact
Correspondence: mihai.netea@radboudumc.nl (M.G.N.), cbock@cemm.oeaw.ac.at (C.B.)